
On March 5th, the InSilc project will host a workshop on In Silico Clinical Trials Concepts and Adoption. This workshop will provide an overview to the InSilc Platform to prospective users by discussing a range of InSilico scenarios that have been completed by the project partners. The workshop runs from 14:00 - 20:00 CET (8:00 AM - 2:00 PM EST).
Dr. Elazer Edelman, Director, Institute for Medical Engineering and Science, MIT and CBSET board chairman will be presenting "The evolution/revolution of in-silico medicine" at 16:05 PM CET (10:05 AM EST). The full agenda is available here:
Presentations of interest:
- 14:15 CET (8:15 AM EST): In Silc - an In Silico platform for drug-eluting BVS design, development and evaluation, Georgia Karanasiou, Senior Researcher, InSilc Coordination Team
- 16:05 CET (10:05 AM EST): The evolution/revolution of In Silico medicine, Dr. Elazer Edelman, Director, Institute for Medical Engineering and Science, MIT; Edward J. Poitras Professor in Medical Engineering and Science, MIT; Professor of Medicine, Harvard Medical School; Senior Attending Physician, Brigham and Women's Hospital; and CBSET board chairman
- 17:05 CET (11:05 AM EST): Towards the adoption of In Silico medicine: the clinical researcher point of view, Robert Byrne, Director of Cardiology Mater Private Hospital, Dublin; Chair of Cardiovascular Research, Royal College of Surgeons
- 17:30 CET (11:30 AM EST): Benefits and challenges for using CM&S for medical device development and clinical trials, David Flynn, Research Fellow, Global Technology Services, CM&S Center of Excellence, Boston Scientific
- 17:45 CET (11:45 AM EST): Adoption of In Silico medicine: use and barriers of M&S in a Medical Technology Company, Markus Reiterer, Dist. Scientist, Medtronic
- 18:00 CET (12:00 PM EST): Adoption of In Silico Design Verification/Validation Testing in the Medical Device Industry, Dejan Krsmanovic, Founder & President, CardioMed Technology
If you would like to attend the InSilc workshop "In Silico Clinical Trials Concepts and Adoption," go here.
Related CBSET Services:
Related CBSET scientific papers & presentations:
Design and implementation of in silico clinical trial for Bioresorbable Vascular Scaffolds. Georgia S Karanasiou, Panagiota I Tsobou, Nikolaos S Tachos, Luca Antonini, Lorenza Petrini, Giancarlo Pennati, Frank Gijsen, Farhad Rikhtegar Nezami, Rami Tzafriri, Ted Vaughan, Martin Fawdry, Dimitrios I Fotiadis. Annu Int Conf IEEE Eng Med Biol Soc. 2020 Jul;2020:2675-2678. doi: 10.1109/EMBC44109.2020.9176317.
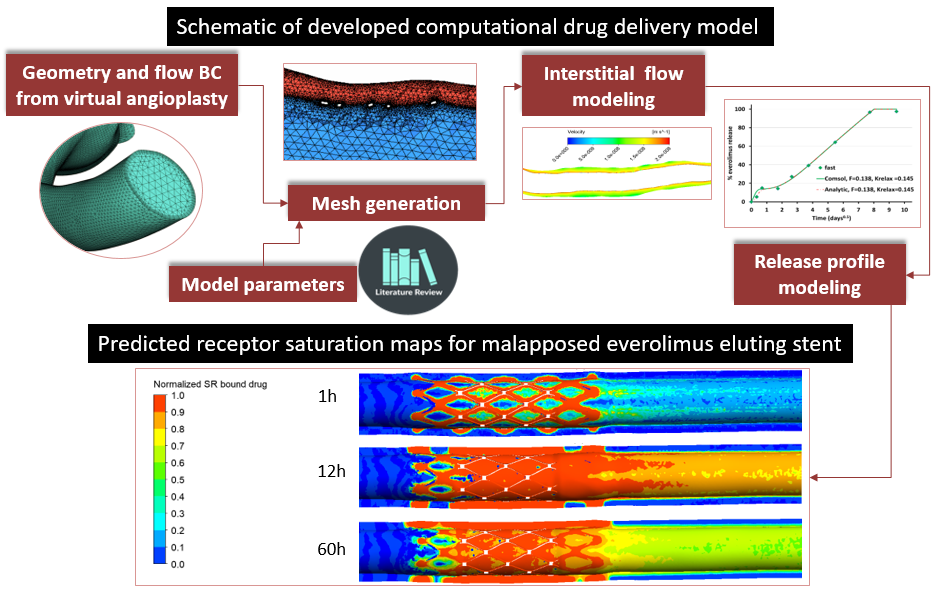
InSilc is a Cloud-based platform integrating beyond the state of the art in silico multiscale and multidisciplinary models towards the delivery of optimized and advanced prediction of short and medium/long term Bioresorbable Vascular Scaffolds (BVS) performance. The InSilc platform covers all phases of product development and testing and its development is realized through a generous grant from the European Union’s HORIZON 2020 research and innovation program.
Led by Drs. Rami Tzafriri and Farhad Nezami, the CBSET/MIT team developed the Drug Delivery Module and designed animal experiments for supporting this and other modules.



