Teigen C, Stanley JR, Johnsont P, Gross C. Preclinical evaluation of the InCraft® aortic endograft in a sheep model.
Summary: Animal models remain the gold standard for the preclinical evaluation of tissue response, sealing and integrity of aortic endografts. Preclinical testing of the InCraft® device was performed to evaluate these attributes. Through the femoral arteries of eight male crossbred sheep, 22 mm diameter InCraft® Aortic Bifurcate devices were deployed in the abdominal aortas, and shortened 13 mm diameter iliac limbs were deployed in the right iliac arteries. Vessels were excised for radiographic and histopathologic assessment at six months. There were no instances of graft thrombosis, type I endoleak or endograft migration. No fractures of the stents or fixation barbs were observed. There were minimal inflammatory changes on histology, characterized by histiocytes and multinucleated giant cells located along the fabric. The InCraft® device has favorable tissue compatibility and functions well in a sheep model, maintaining patency and sealing without migration, stent fracture or abnormal histologic changes.
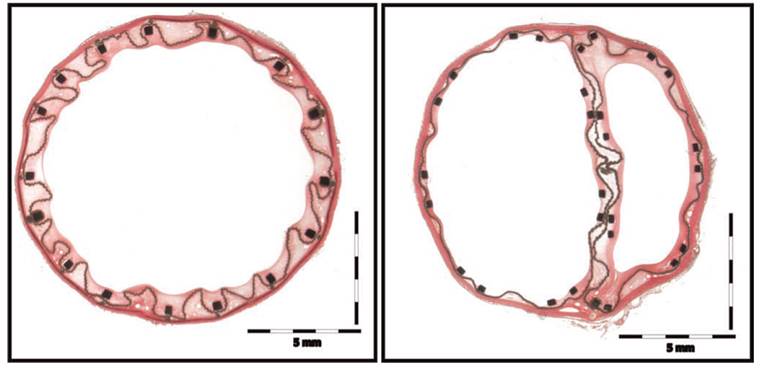
Vascular. 2014 Feb;22(1):13-9.

