How one medtech startup and its preclinical research partner overcame pandemic-related obstacles to continue its medical device research project.
How one medtech startup and its preclinical research partner overcame pandemic-related obstacles to continue its medical device research project.
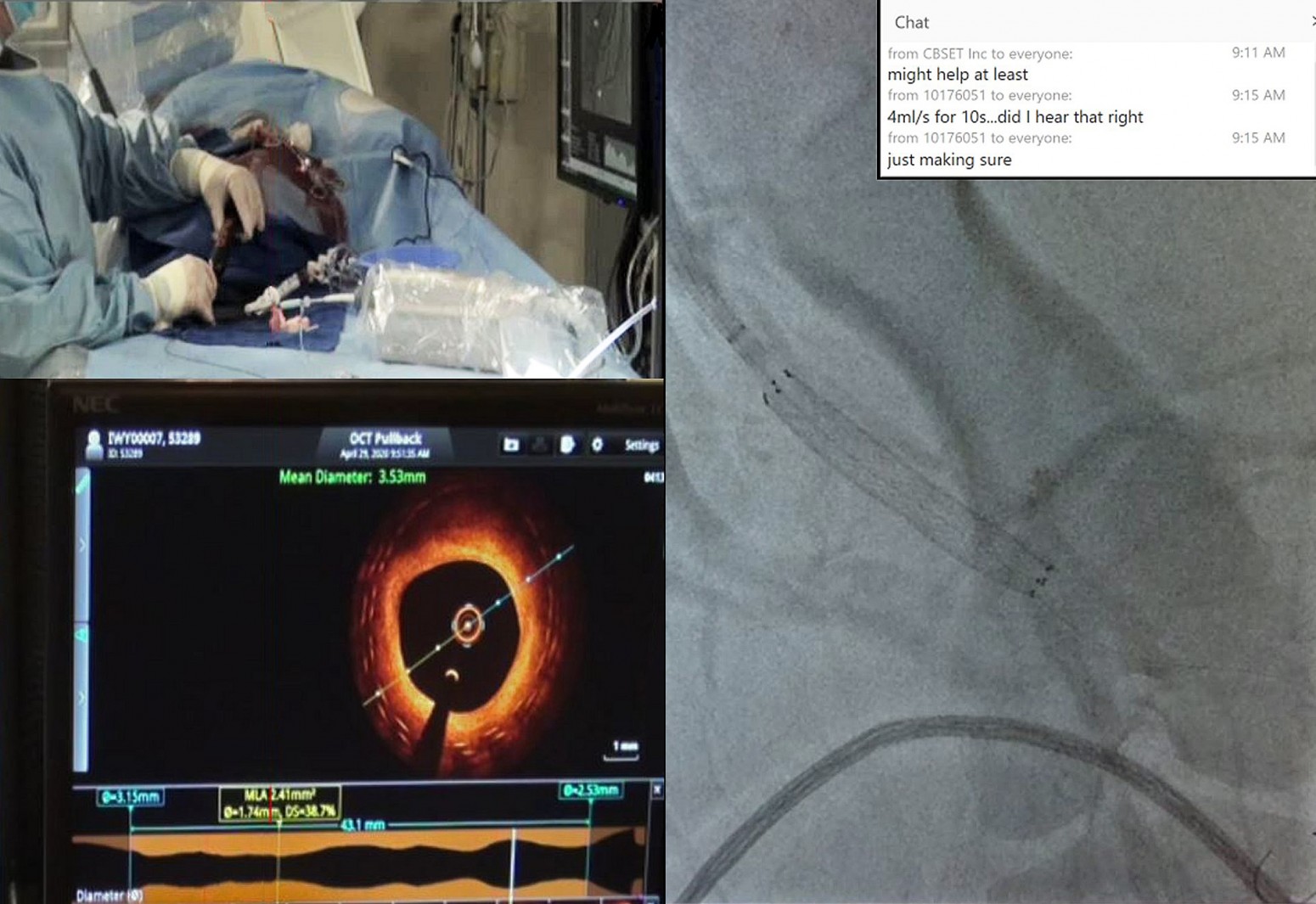
Prior to COVID-19, VentureMed was on a roll. The Minneapolis, MN-based company’s first-generation Flex Vessel Prep System had demonstrated promising clinical performance following FDA clearance and the company was preparing to introduce an improved second-generation device.
To keep the project on track, VentureMed contacted CBSET to see if they could execute a study via livestreaming, as VentureMed personnel and their physician advisors were unable to travel due to COVID-19.
“VentureMed and CBSET both were prepared to adapt and function in this new and challenging environment by continuing development in a more virtual way, modifying operations and building relationships in new ways,” Zeroni said. “At the peak of the uncertainty and shut-downs across the industry, we were able to collaborate with CBSET to execute, complete, and deliver data from a key study. The structure at CBSET, together with the talent and flexibility of the CBSET team, allowed the VentureMed and CBSET teams to design and execute a complex study virtually, and deliver successful results.“