Marie-Pierre Pasdelou, Lise Byelyayeva, Susanna Malmström, Sylvie Pucheu, Marie Peytavy, Hugo Laullier, Donald B. Hodges, Abraham R. Tzafriri, Gaëlle Naert. Ototoxicity: a high risk to auditory function that needs to be monitored in drug development.
The aim of this publication is to raise awareness of drug-induced ototoxicity and to formulate some recommendations based on available guidelines and own experience.
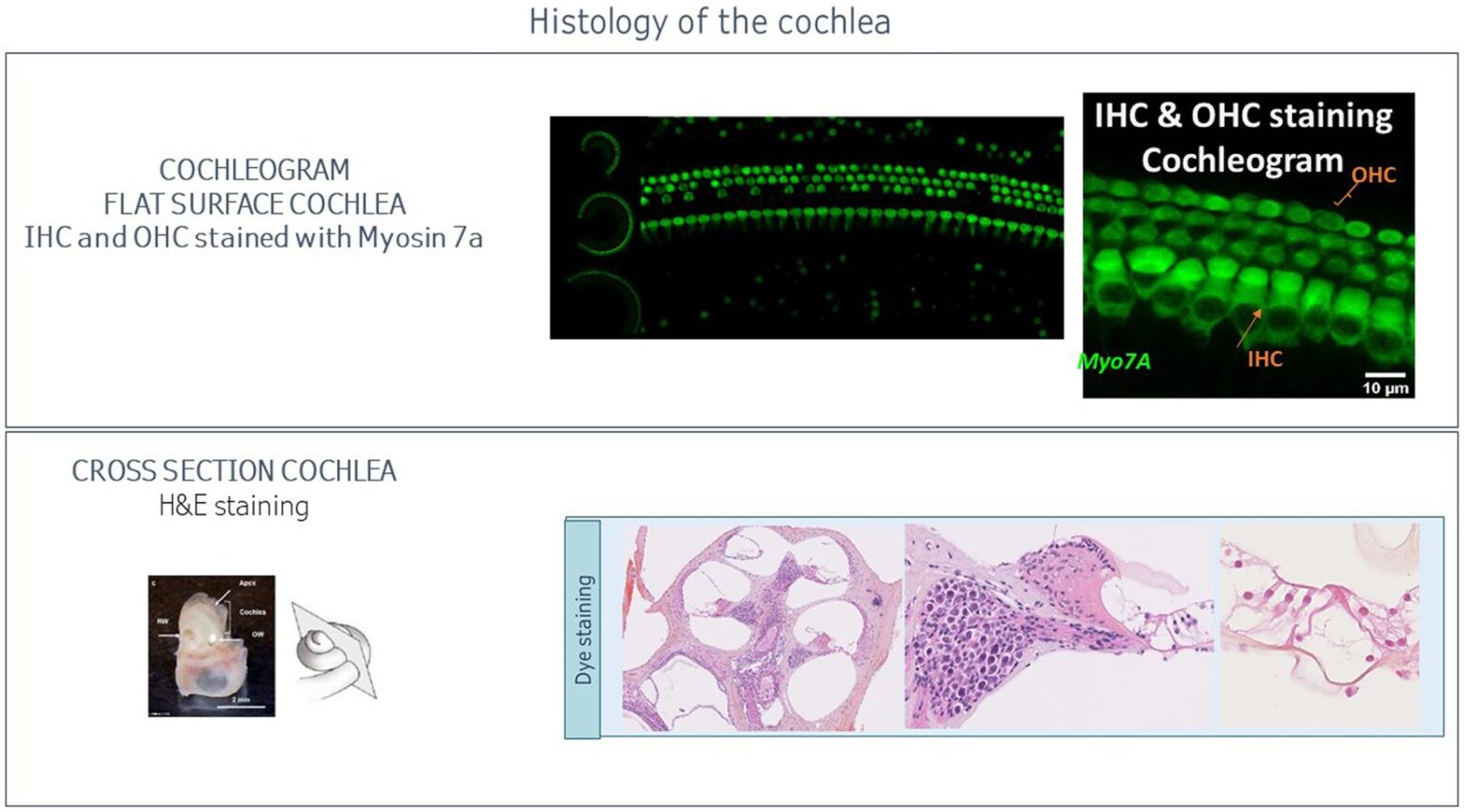 Ototoxicity testing programs should be adapted to the type of therapy, its indication (targeting the ear or part of other medications classes being potentially ototoxic), and the number of assets to test. For multiple molecules and/or multiple doses, screening options are available: in vitro (otic cell assays), ex vivo (cochlear explant), and in vivo (in zebrafish). In assessing the ototoxicity of a candidate drug, it is good practice to compare its ototoxicity to that of a well-known control drug of a similar class. Screening assays provide a streamlined and rapid method to know whether a drug is generally safe for inner ear structures. Mammalian animal models provide a more detailed characterization of drug ototoxicity, with a possibility to localize and quantify the damage using functional, behavioral, and morphological read-outs. Complementary histological measures are routinely conducted notably to quantify hair cells loss with cochleogram.
Ototoxicity testing programs should be adapted to the type of therapy, its indication (targeting the ear or part of other medications classes being potentially ototoxic), and the number of assets to test. For multiple molecules and/or multiple doses, screening options are available: in vitro (otic cell assays), ex vivo (cochlear explant), and in vivo (in zebrafish). In assessing the ototoxicity of a candidate drug, it is good practice to compare its ototoxicity to that of a well-known control drug of a similar class. Screening assays provide a streamlined and rapid method to know whether a drug is generally safe for inner ear structures. Mammalian animal models provide a more detailed characterization of drug ototoxicity, with a possibility to localize and quantify the damage using functional, behavioral, and morphological read-outs. Complementary histological measures are routinely conducted notably to quantify hair cells loss with cochleogram.
Ototoxicity studies can be performed in rodents (mice, rats), guinea pigs and large species. However, in undertaking, or at the very least attempting, all preclinical investigations within the same species, is crucial. This encompasses starting with pharmacokinetics and pharmacology efficacy studies and extending through to toxicity studies. In life read-outs include Auditory Brainstem Response (ABR) and Distortion Product OtoAcoustic Emissions (DPOAE) measurements that assess the activity and integrity of sensory cells and the auditory nerve, reflecting sensorineural hearing loss.
Accurate, reproducible, and high throughput ABR measures are fundamental to the quality and success of these preclinical trials. As in humans, in vivo otoscopic evaluations are routinely carried out to observe the tympanic membrane and auditory canal. This is often done to detect signs of inflammation. The cochlea is a tonotopic structure. Hair cell responsiveness is position and frequency dependent, with hair cells located close to the cochlea apex transducing low frequencies and those at the base transducing high frequencies. The cochleogram aims to quantify hair cells all along the cochlea and consequently determine hair cell loss related to specific frequencies. This measure is then correlated with the ABR & DPOAE results
 Ototoxicity assessments evaluate the impact of drug candidates on the auditory and vestibular systems, de-risk hearing loss and balance disorders, define a safe dose, and optimize therapeutic benefits. These types of studies can be initiated during early development of a therapeutic solution, with ABR and otoscopic evaluations. Depending on the mechanism of action of the compound, studies can include DPOAE and cochleogram. Later in the development, a GLP (Good Laboratory Practice) ototoxicity study may be required based on otic related route of administration, target, or known potential otic toxicity.
Ototoxicity assessments evaluate the impact of drug candidates on the auditory and vestibular systems, de-risk hearing loss and balance disorders, define a safe dose, and optimize therapeutic benefits. These types of studies can be initiated during early development of a therapeutic solution, with ABR and otoscopic evaluations. Depending on the mechanism of action of the compound, studies can include DPOAE and cochleogram. Later in the development, a GLP (Good Laboratory Practice) ototoxicity study may be required based on otic related route of administration, target, or known potential otic toxicity.
By elucidating the mechanisms through which ototoxic drugs damage the auditory system, Cilcare and CBSET authors highlight the importance of understanding the pathophysiology of drug-induced hearing loss andadvocate for integrating advanced screening technologies and predictive models into the drug development process to mitigate potential risks to auditory health.

