Jessica L. Grimsby, Matthew D. Szkolnicki, Kevin A. Wood. Reevaluation of an Established In Vivo Gastric Vessel Bleed Model for Hemostatic Device Safety and Efficacy Testing
This study was overseen by the CBSET Inc. Contract Research Organization’s (Lexington, MA, USA.) Institutional Animal Care and Use Committee (IACUC) and conformed to the “Guide for the Care and Use of Laboratory Animals”. CBSET, Inc. is accredited by AAALAC International and is registered with the U.S. Department of Agriculture.
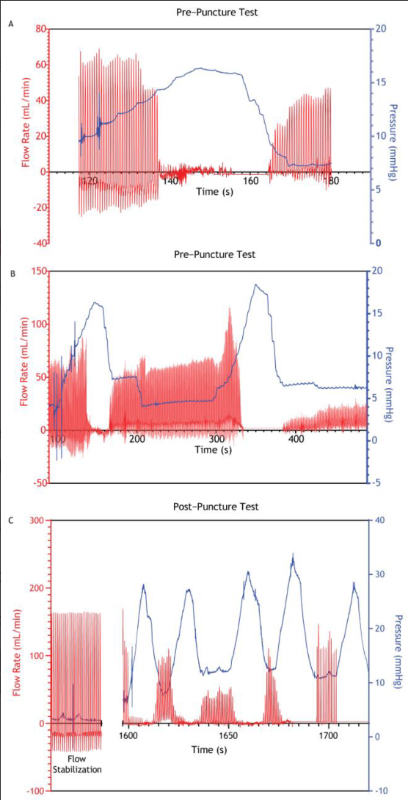 Background: For over a decade, endoscopic hemostatic powders have been used to manage upper gastrointestinal bleeding (UGIB). Various preclinical benchtop and animal models have been developed to evaluate these devices. Multiple companies have released hemostatic powders to market, assessing their safety and efficacy using an established porcine gastric vessel bleed model. The model requires inserting an artery segment into the gastric lumen, which is then punctured to produce a bleed. This simulates an aggressive arterial bleed, allowing hemostatic prototype devices to be tested under challenging conditions.
Background: For over a decade, endoscopic hemostatic powders have been used to manage upper gastrointestinal bleeding (UGIB). Various preclinical benchtop and animal models have been developed to evaluate these devices. Multiple companies have released hemostatic powders to market, assessing their safety and efficacy using an established porcine gastric vessel bleed model. The model requires inserting an artery segment into the gastric lumen, which is then punctured to produce a bleed. This simulates an aggressive arterial bleed, allowing hemostatic prototype devices to be tested under challenging conditions.
Methods: We aimed to evaluate the relationship between intragastric pressure and bleed severity by injecting the gas used to deliver hemostatic powder to the bleed site without administering the hemostatic powder.
Results: Our results indicate that elevated intragastric pressures alone can cause bleed cessation. Additional findings suggest that other factors in the model can lead to false positive hemostasis.
Conclusions: This study highlights limitations in the current state porcine gastric vessel bleed model. The results underscore the importance of vetting preclinical models before acquiring efficacy data and the need to develop more robust and effective bleed models for testing hemostatic devices.

