Supporting drug and cell-based therapies throughout the preclinical continuum
CBSET’s collaborative approach supports preclinical research from proof-of-concept through regulatory filing, on projects ranging from simple ad hoc studies, through IND-enabling GLP studies. We were founded by pioneers in novel therapeutic approaches and include noted scientists in regenerative medicine among our research staff.
Common project aims
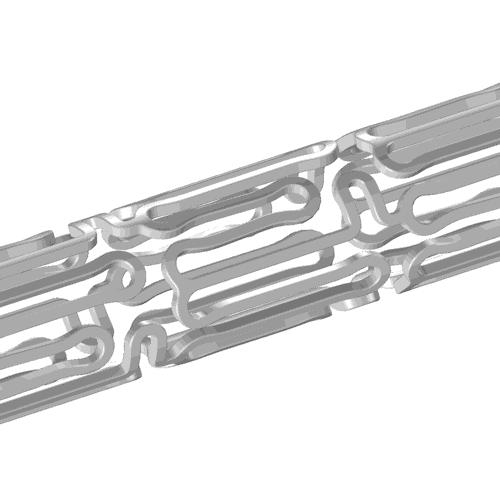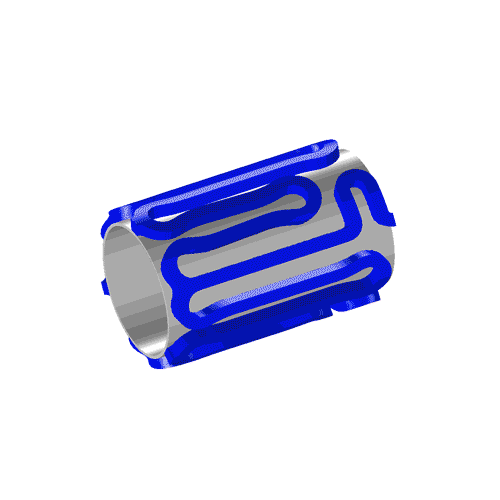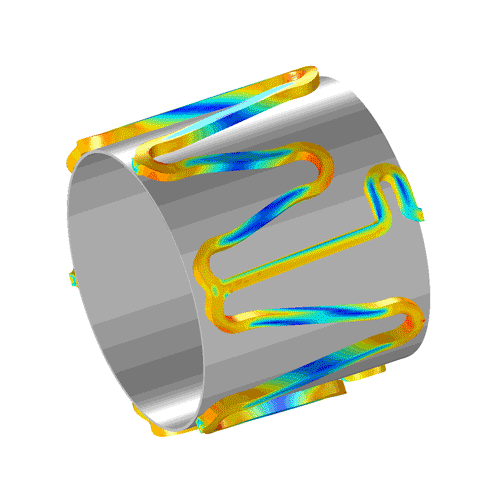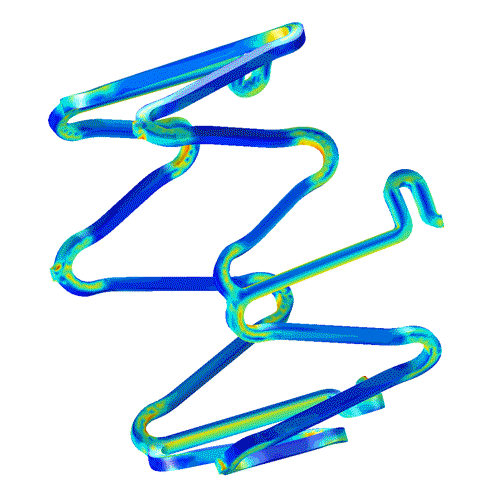
Characterize your small molecule or biologic with high-quality collaborative GLP studies in large and small animals, including:
- Efficacy and safety
- Delivery paradigms
- Translatability
- Complex pathologies
- Regulatory strategy
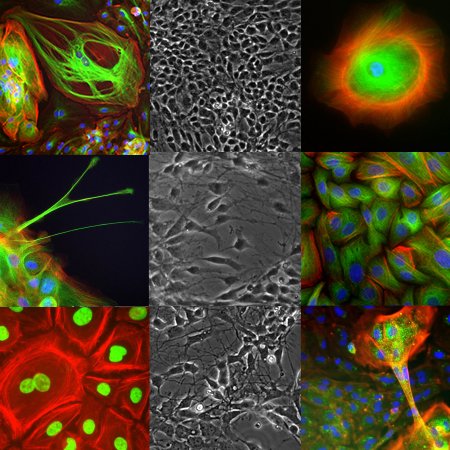 Cell-based therapeutics
Cell-based therapeutics
Develop efficient testing paradigms with experts in regenerative medicine
Cell-based medicines are being devised for every therapeutic area, each a novel regulatory challenge. CBSET has a long history of success in supporting studies in regenerative medicine. Our institute assimilates in-life procedures in animal models, ex vivo analyses and complex pathologies in a cohesive collaboration.
- Integrate lab animal sciences with physical modeling and pathology
- Profile cell persistence and distribution
- Approach the pre-IND with a credible partner
- Develop testing paradigms with experts in regenerative medicine
Localized drug delivery and kinetics
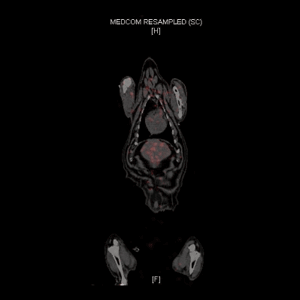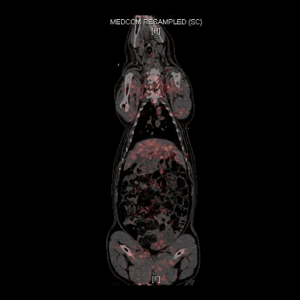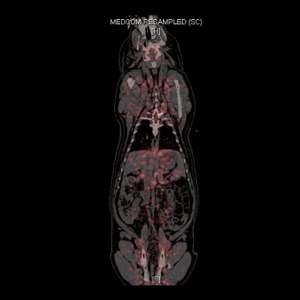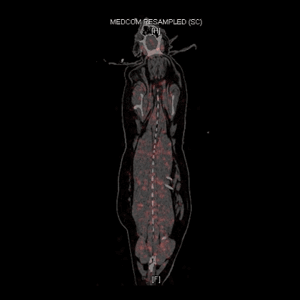 Integrate lab animal sciences with physical modeling and pathology...
Integrate lab animal sciences with physical modeling and pathology...
CBSET's multi-disciplinary approach can integrate computational modeling of drug release, absorption, and local tissue distribution. We pair the complexities of anatomic and surgical methods with a deep understanding of drug kinetics to meet your needs in a variety of applications, including:
- Pharmacokinetics (PK) / bio-distribution
- Drug-material interactions and formulation
- Computational and 3D modeling
- Controlled-release therapeutics
- Combination drug-device products
...in one multi-disciplinary research institute.
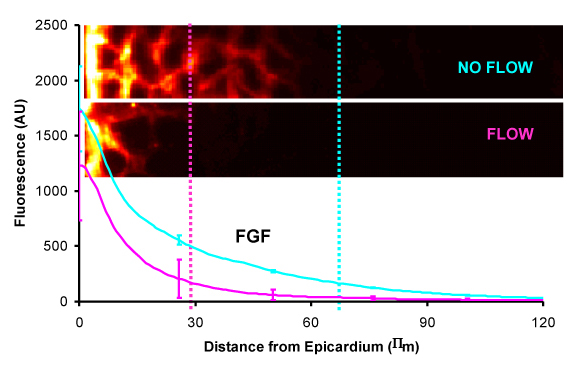
- On-site tissue culture capability
- Open paradigm of collaboration as a non-profit (501 C3) institute
- Operational compliance and accreditation - GLP, OLAW, AAALAC
- Surgical methods - on-site operating suites and multiple imaging modalities
- Animal Sciences - full-time ACLAM-certified DVM
- Experts in safety - full-time boarded DACVP
- Full histology suite for all phases of embedding, sectioning, staining and imaging (IHC, IF)